| Description | Potassium cyanide is an inorganic compound with the formula KCN. This
colorless crystalline compound, similar in appearance to sugar, is highly
soluble in water. Most KCN is used in gold mining, organic synthesis, and
electroplating. Smaller applications include jewelry for chemical gilding
and buffing.
KCN is highly toxic. The moist solid emits small amounts of hydrogen
cyanide due to hydrolysis, which tastes like bitter almonds. Not everyone,
however, can taste this: the ability to do so is a genetic trait.
It is used by entomologists as a killing agent in collecting jars, as most
insects succumb within seconds, minimizing damage of even highly fragile
specimens.
KCN is produced by treating hydrogen cyanide with a 50% aqueous solution of
potassium hydroxide, followed by evaporation of the solution in a vacuum:
HCN + KOH ? KCN + H2O
or by treating formamide with potassium hydroxide:
HCONH2 + KOH ? KCN + 2H2O
Approximately 50,000 tons of potassium cyanide are produced yearly
EMAIL..... brightsidepharma(@)protonmail(.)com
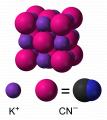
In aqueous solution, KCN is dissociated into hydrated K+ ions and CN-. As a
solid, the salt crystallizes such that the cations and anions organize like
Na+ and Cl- in NaCl. The cations and anions six-coordinate. Each K+ is
linked to two pi-bonds of the CN- as well as two links each to C and N
each. Since CN- is diatomic, the symmetry of the solid is lower than that
in NaCl. The cyanide anions form sheets. The CN- ions rapidly rotate in the
solid at ambient temperature such that the time-averaged shape of the CN-
ions is spherical
|
 United States
United States